For Healthcare Professionals Outside the US
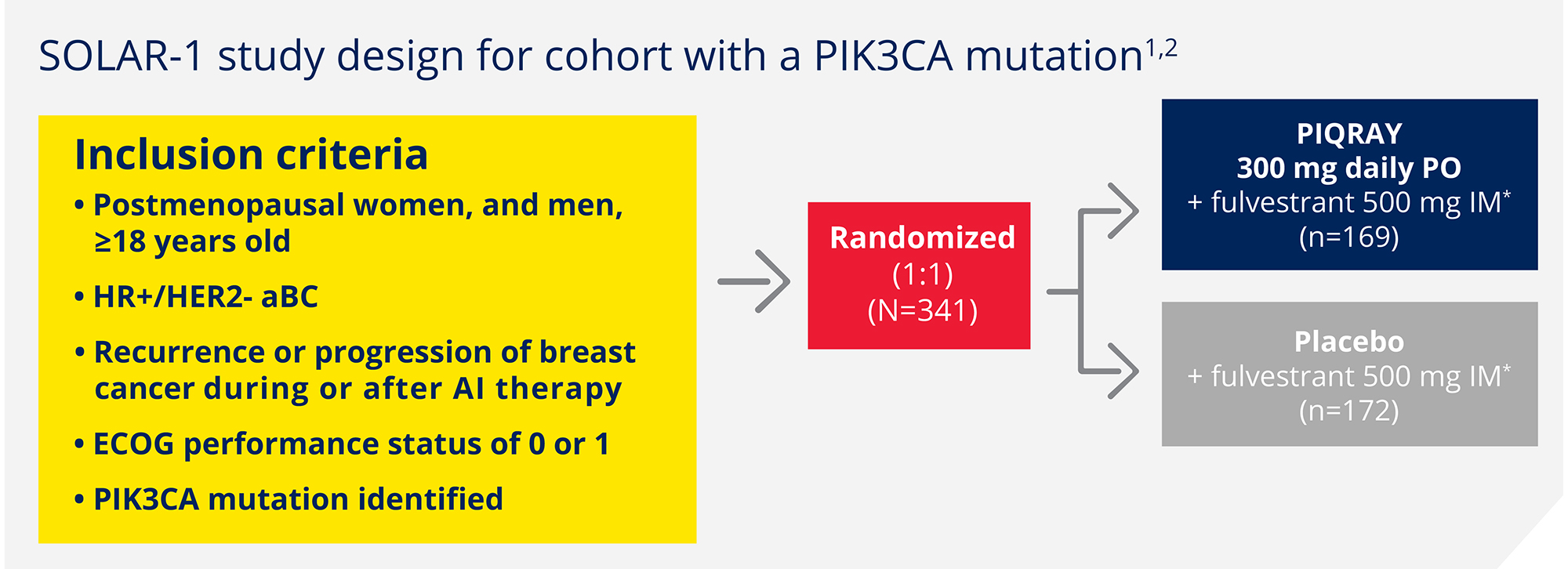
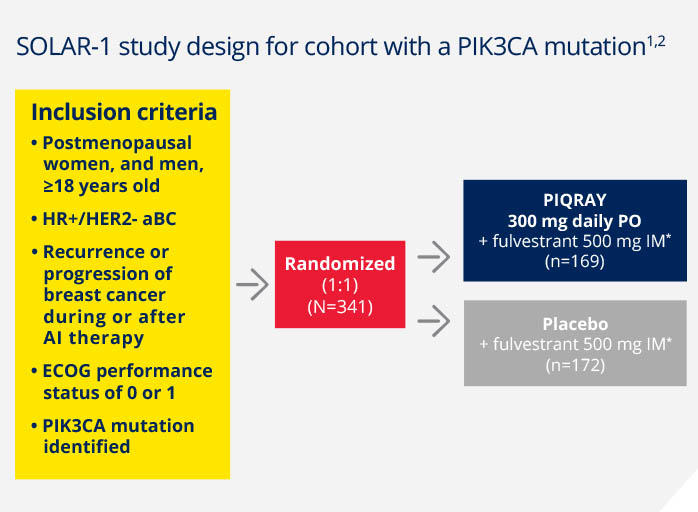
First phase 3 trial leading to an approval specifically for aBC patients with a PIK3CA mutation1
Randomized, double-blind, prospective, placebo-controlled trial in HR+/HER2- aBC1,2
A total of 572 patients were enrolled in the
trial, including 341 patients with a PIK3CA
mutation
Patients were stratified by:
- Presence of liver and/or lung metastases
- Prior CDK4/6 inhibitor treatment
A total of 231 patients did not have a PIK3CA
mutation and were included as a separate cohort
as a proof of concept to evaluate PIQRAY as a
biomarker-driven therapy. The proof of concept
criteria were not met for this cohort.2
Primary endpoint1,2
Progression-free survival (PFS) in patients with a PIK3CA mutation (investigator assessment)
Key secondary endpoint1,2
Overall survival in patients with a PIK3CA mutation
Additional secondary endpoints1-3
Overall response rate in patients with a PIK3CA mutation
Clinical benefit rate in patients with a PIK3CA mutation
Safety
Health-related quality-of-life (HRQOL)
*Fulvestrant given on day 1 and day 15 of the first 28-day cycle, then day 1 of subsequent 28-day cycles.
Patients with a broad range of characteristics were represented1,2
Baseline characteristics of patients with a
PIK3CA mutation2,3
| Characteristica |
PIQRAY + fulvestrant (n=169)b |
Plabeco + fulvestrant (n=172)c |
|---|---|---|
| Line of advanced anticancer treatmentd | ||
| First line | 88 (52.1) | 89 (51.7) |
| Second line | 79 (46.7) | 82 (47.7) |
| Median age, years (range) | 63 (25-87) | 64 (38-92) |
| Race | ||
| Caucasian | 117 (69.2) | 109 (63.4) |
| Asian | 34 (20.1) | 40 (23.3) |
| Other/unknown | 18 (10.7) | 23 (13.4) |
| ECOG performance status | ||
| 0 | 112 (66.3) | 113 (65.7) |
| 1 | 56 (33.1) | 58 (33.7) |
| Metastatic sites | ||
| Visceral disease | 93 (55.0) | 100 (58.1) |
| Lung metastasis | 57 (33.7) | 68 (39.5) |
| Liver metastasis | 49 (29.0) | 54 (31.4) |
| Bone-only disease | 42 (24.9) | 35 (20.3) |
| Prior chemotherapy | ||
| Neoadjuvant | 25 (14.8) | 29 (16.9) |
| Adjuvant | 78 (46.2) | 86 (50.0) |
| Prior CDK4/6 inhibitor treatment | 9 (5.3) | 11 (6.4) |
bOne man was enrolled in the PIQRAY + fulvestrant arm. All other study participants were postmenopausal women.
cOne patient randomized to placebo was not treated.
dIn the SOLAR-1 study, first line was defined as patients whose disease progressed >1 year after (neo)adjuvant endocrine therapy (ET) or
whose disease progressed >1 year after (neo)adjuvant ET, and who did not receive prior treatment for aBC. Second line was defined as patients
whose disease progressed >1 year after (neo)adjuvant ET and while on or after one line of ET for aBC or patients with newly diagnosed aBC
whose disease progressed while on or after one line of ET.
References:
1. Piqray®
(alpelisib)
EU Summary of Product Characteristics. Novartis;
2022.
2. André F,
Ciruelos E,
Rubovszky G,
et al. Alpelisib
for PIK3CA-mutated, hormone receptor-positive
advanced breast cancer.
N Engl J Med. 2019;380(20):1929-1940.
3. Data on file.
Novartis Pharmaceuticals Corp; 2018.
This is an international site for PIQRAY®
(alpelisib) and is intended for healthcare professionals
outside the US.