For Healthcare Professionals Outside the US
PIQRAY + fulvestrant nearly doubled mPFS in patients with a PIK3CA mutation1,2
PFS in patients with a PIK3CA mutation1,2
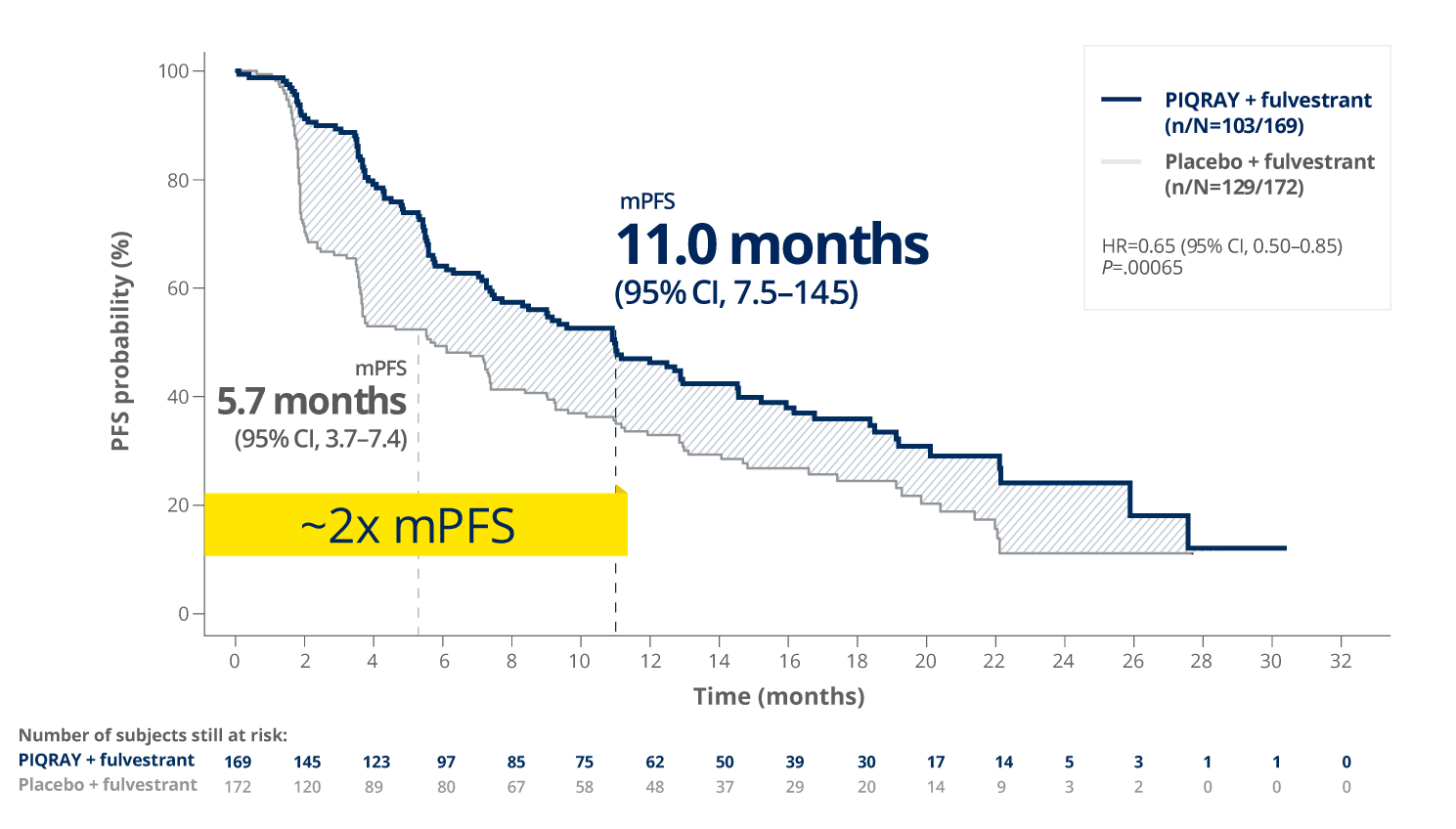
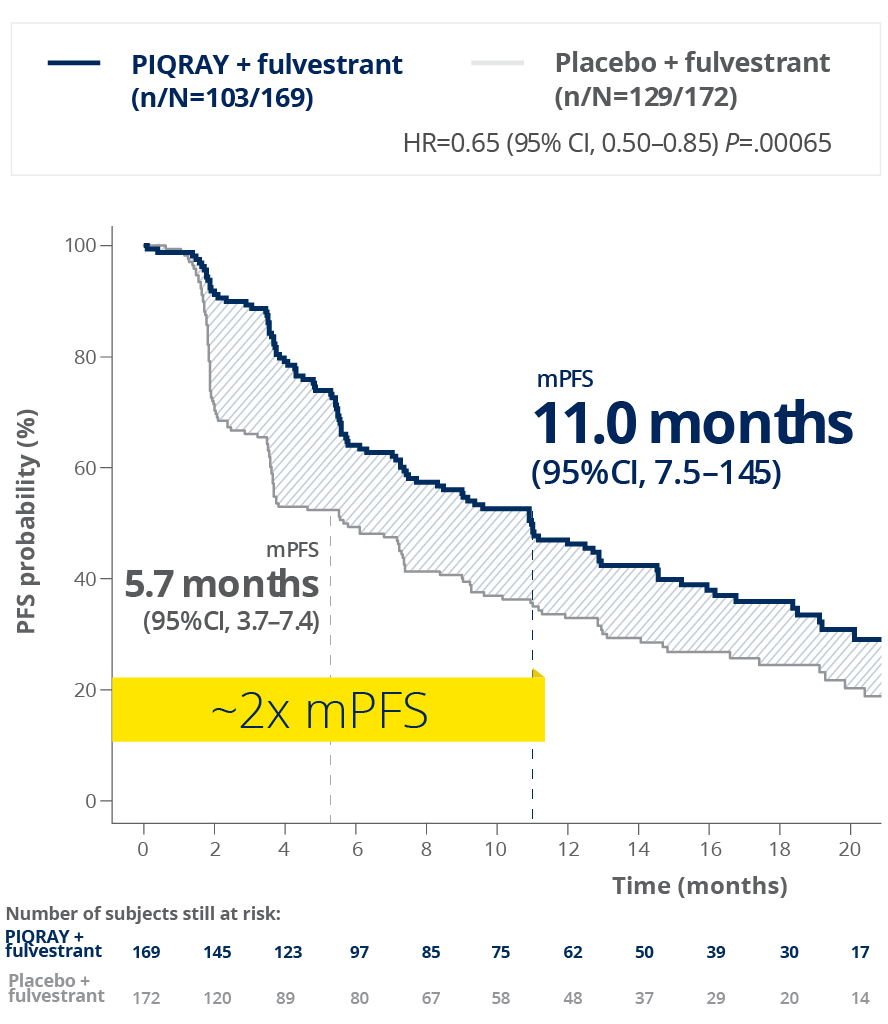
Results reported at 2 months were not
prespecified and are observational in nature; as
such, there was no prespecified statistical
procedure controlling for type 1 error
Rapid separation of PFS curve evident at 2 months1,2
Approximately 8 months of OS benefit3
- Median OS was 39.3 months (95% CI, 34.1-44.9) for PIQRAY + fulvestrant vs 31.4 months (95% CI, 26.8-41.3) for placebo + fulvestrant (HR=0.86; [95% CI, 0.64-1.15] P=.15)
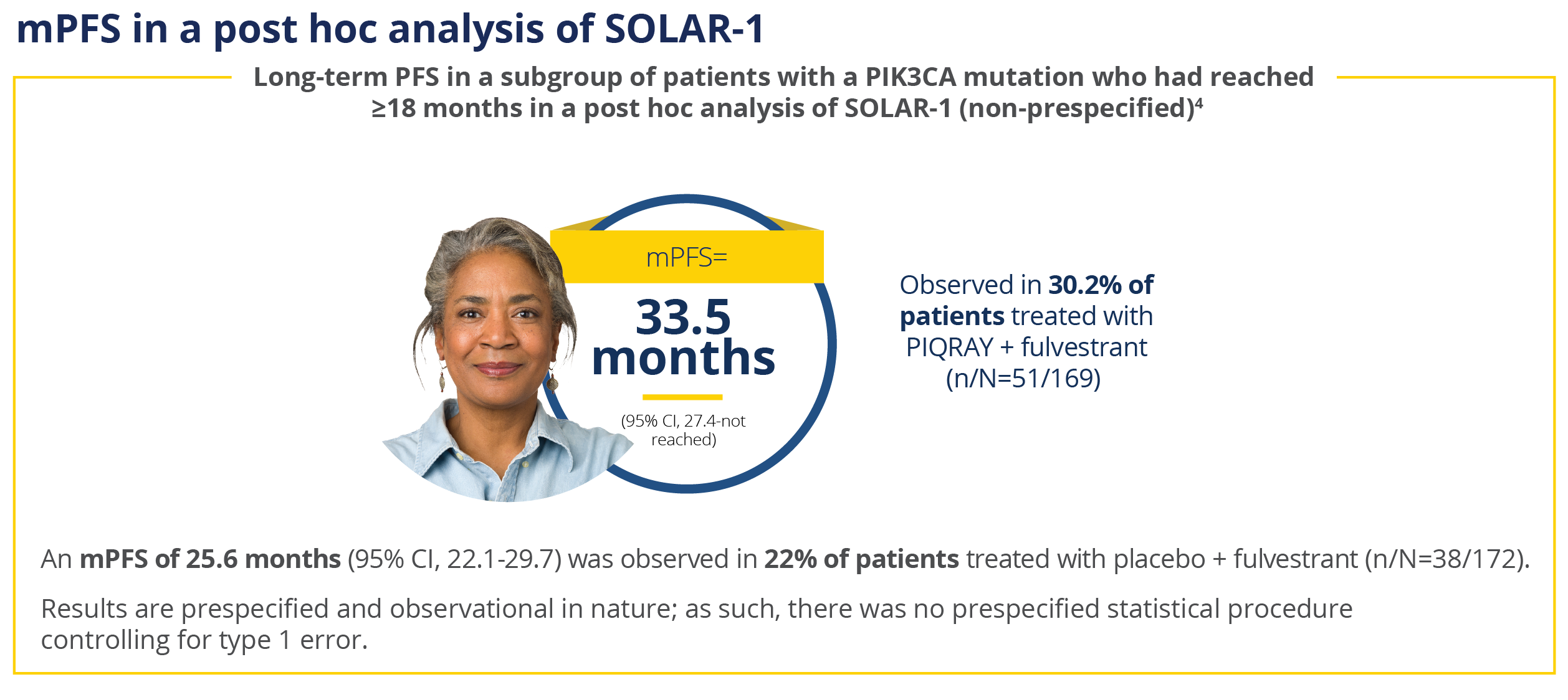
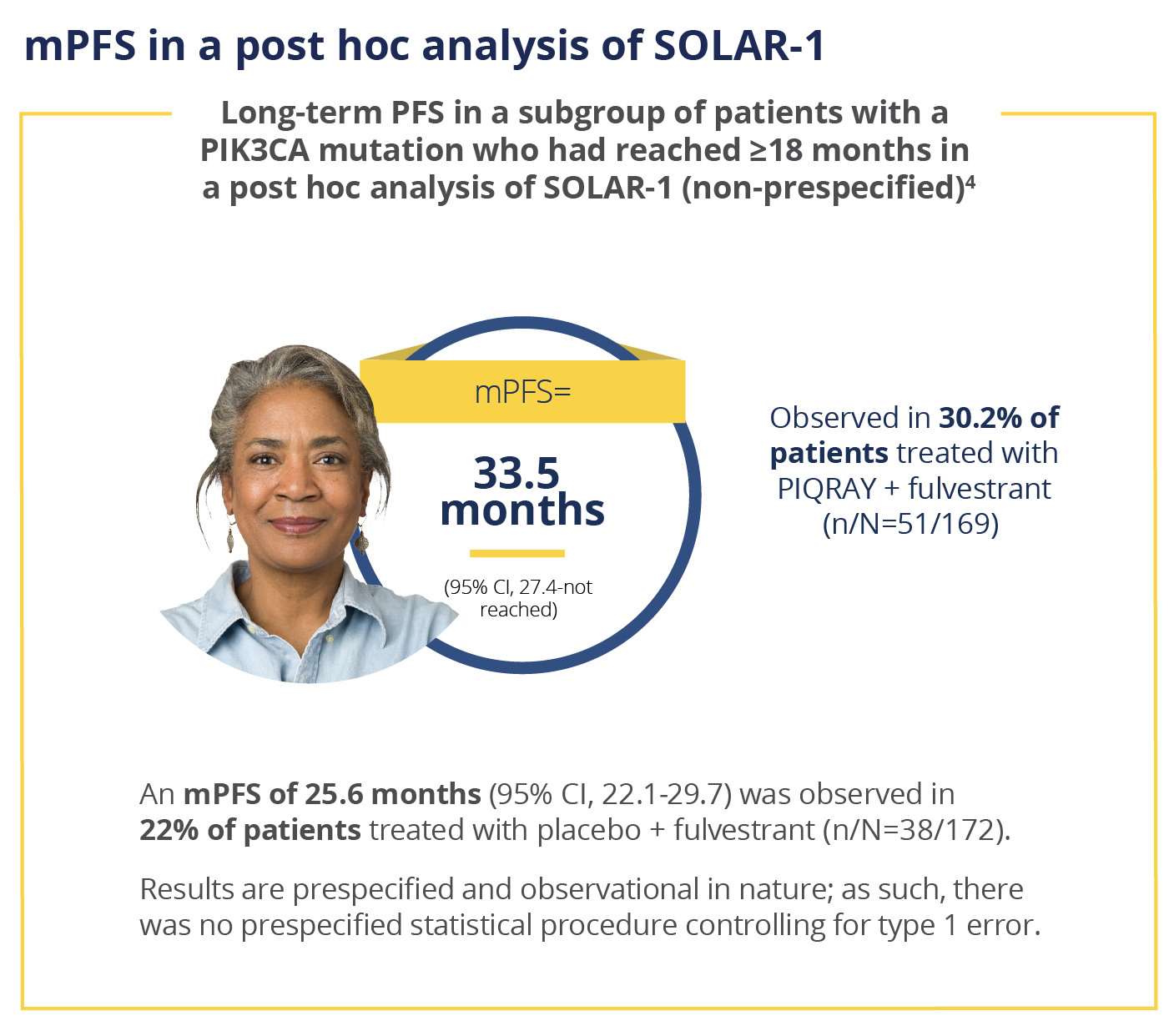
mPFS, median progression-free survival; PFS,
progression-free survival.
Consistent PFS results across subgroups1,2
PFS in select patient subgroups with a PIK3CA
mutation2
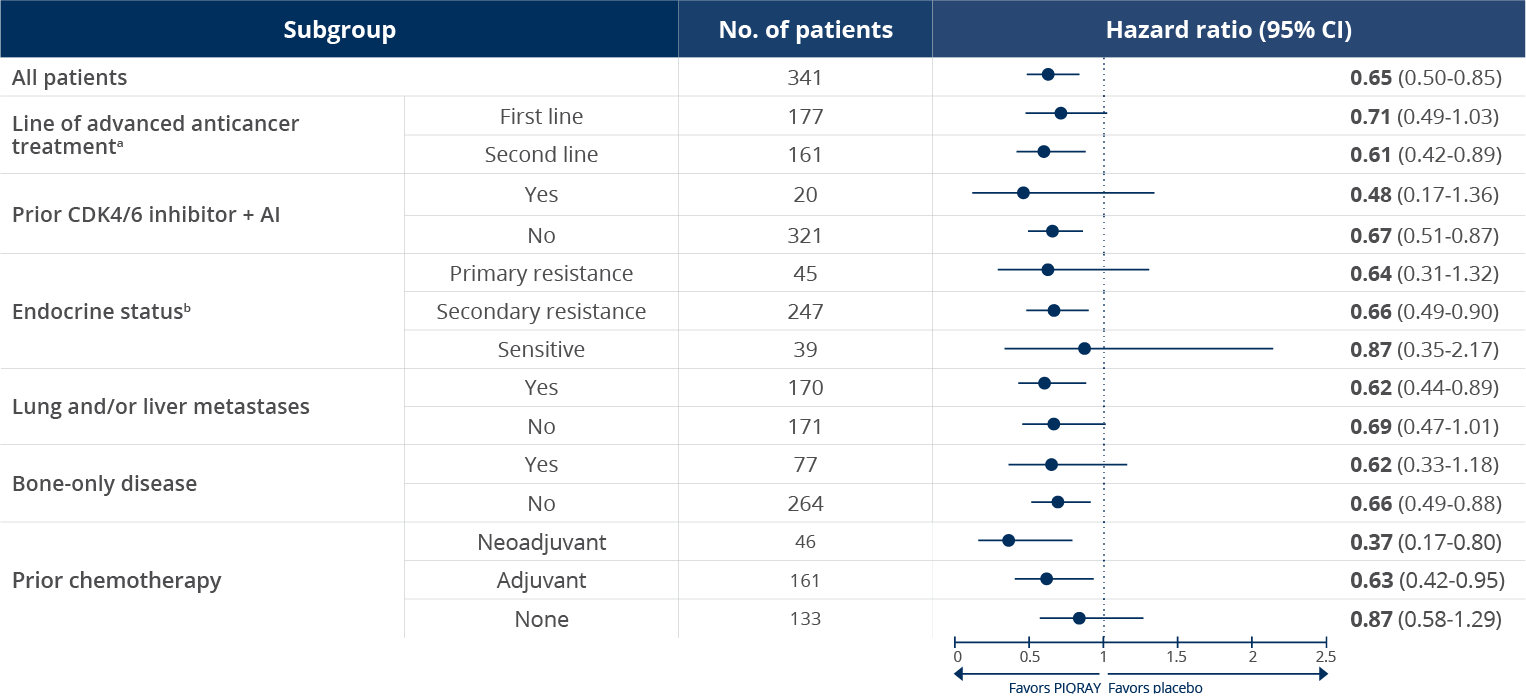
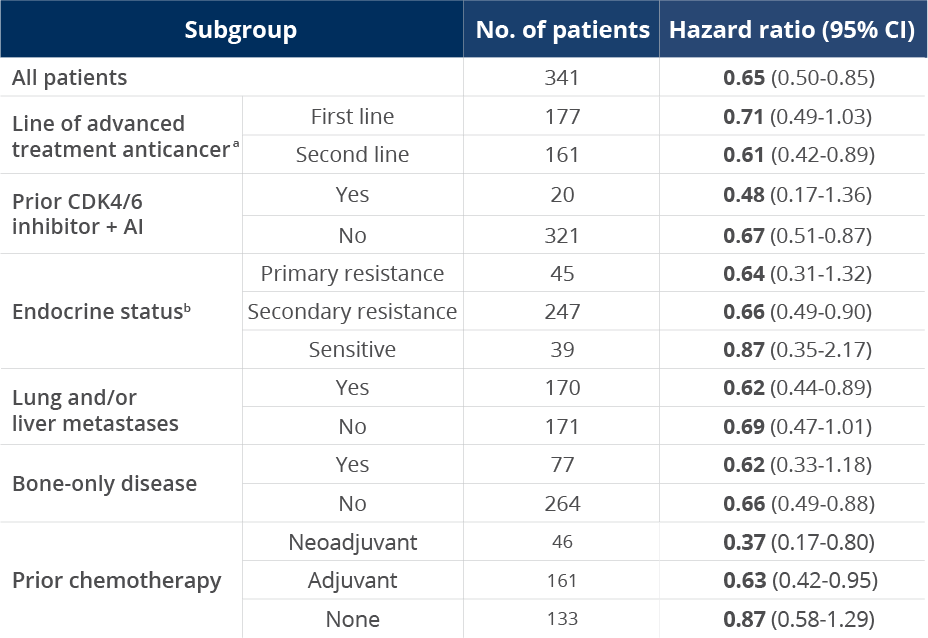
The data are from prespecified subgroup
analyses of the primary endpoint (mPFS) in
SOLAR-1 and are not powered to detect
statistical significance
aIn the SOLAR-1
study, first line was defined as patients whose
disease progressed ≤1 year after
(neo)adjuvant ET or whose disease progressed >1
year after (neo)adjuvant ET, and who did not
receive prior treatment for aBC. Second line was
defined as patients whose disease progressed >1
year after (neo)adjuvant ET and while on or
after one line of ET for aBC or patients with
newly diagnosed aBC whose disease progressed
while on or after one line of ET.
bIn the SOLAR-1 study, primary endocrine resistance was defined as relapse within 24 months on adjuvant ET or progression within 6 months on ET for advanced disease. Secondary endocrine resistance was defined as relapse after 24 months on adjuvant ET, relapse within 12 months of the end of adjuvant ET, or progression after 6 months on ET for advanced disease. Endocrine sensitive was defined as relapse ≥12 months after completion of ET in the adjuvant setting.3
bIn the SOLAR-1 study, primary endocrine resistance was defined as relapse within 24 months on adjuvant ET or progression within 6 months on ET for advanced disease. Secondary endocrine resistance was defined as relapse after 24 months on adjuvant ET, relapse within 12 months of the end of adjuvant ET, or progression after 6 months on ET for advanced disease. Endocrine sensitive was defined as relapse ≥12 months after completion of ET in the adjuvant setting.3
More than doubled the response rate1,2
ORR in patients with a PIK3CA mutation who had
measurable disease1,2
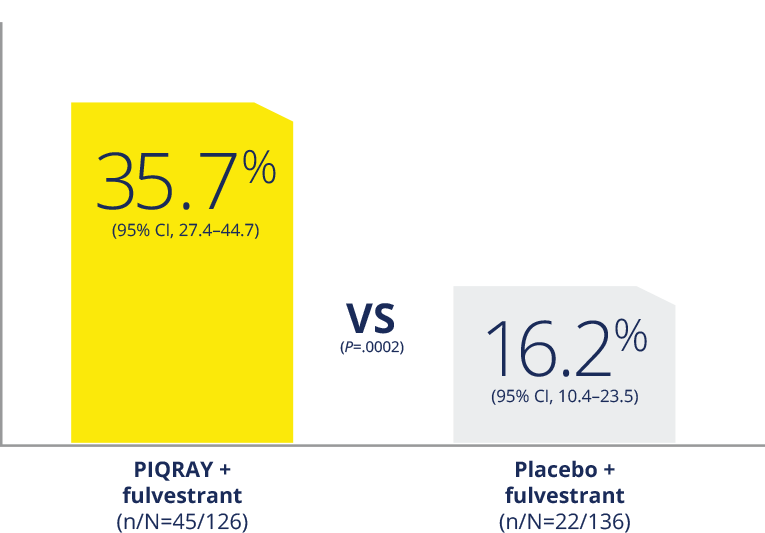

ORR in all patients with a PIK3CA
mutation2
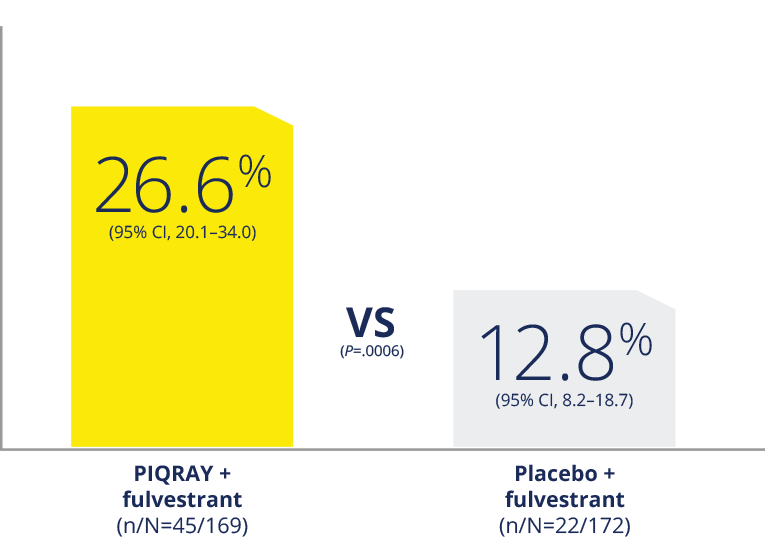
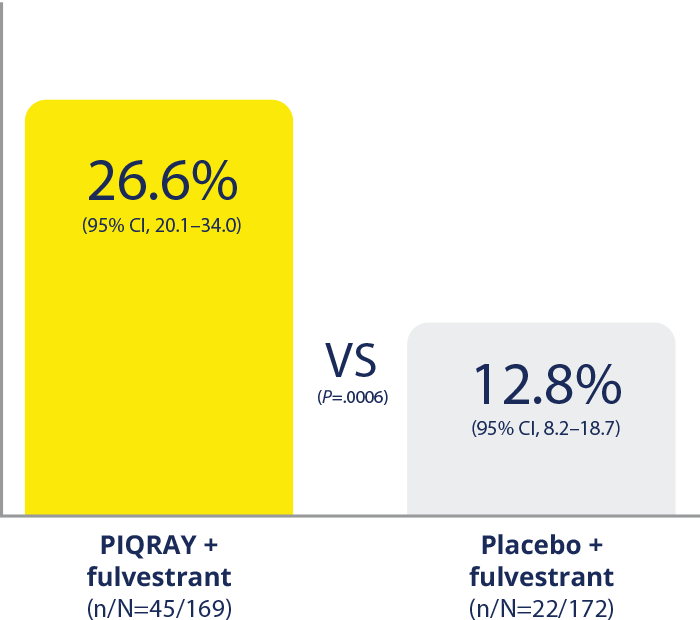
ORR was defined as the percentage of subjects
with confirmed complete response or partial
response. Measurable disease was defined as the
presence of at least one measurable nodal or
non-nodal lesion as per RECIST v1.1 criteria.
ORR, overall response rate.
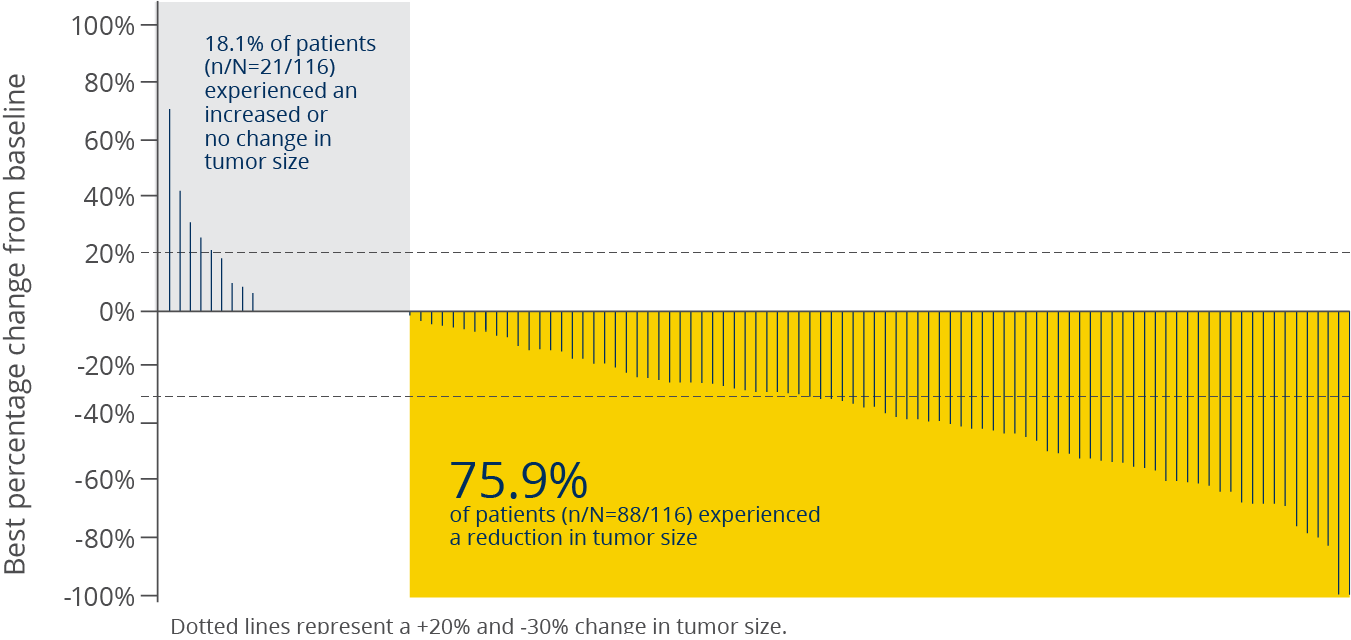
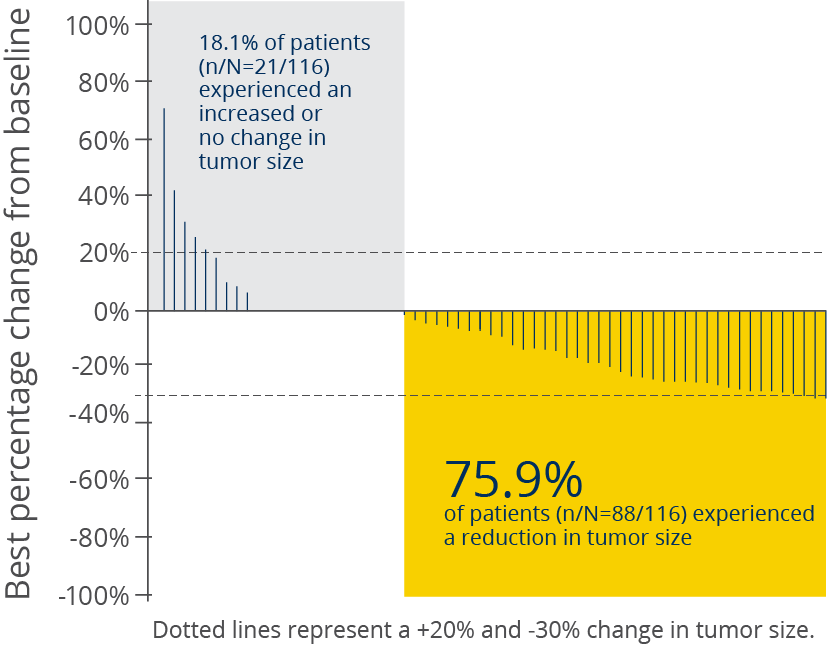
Tumor shrinkage was observed in 3 out of 4 patients with a PIK3CA mutation5
Best percentage change in tumor size in patients
with a PIK3CA mutation5
Additional results
 Similar EORTC QLQ-C30 global health
status/QoL scores in both arms in
the PIK3CA mutant cohort6
Similar EORTC QLQ-C30 global health
status/QoL scores in both arms in
the PIK3CA mutant cohort6
There was no statistical difference between the two treatment arms in time to 10% deterioration (TTD) in EORTC QLQ-C30 global health/QoL status (HR=1.03; 95% CI, 0.72-1.48)*
 Delayed time to chemotherapy (TTC)
by 8.5 months3
Delayed time to chemotherapy (TTC)
by 8.5 months3
PIQRAY + fulvestrant delayed median TTC by 8.5 months vs placebo + fulvestrant (23.3 months vs 14.8 months; HR=0.72 [95% CI, 0.54-0.95]) which is meaningful to patients
*TTD in global health status EORTC QLQ-C30 was defined as time between baseline and first occurrence of ≥10 point worsening of global health status (EORTC QLQ-C30 global health scale score) compared to baseline with no later improvement above this threshold observed during the treatment period or death due to any cause.
References:
1. Piqray®
(alpelisib)
EU Summary of Product Characteristics. Novartis;
2022.
2. André F,
Ciruelos E,
Rubovszky G,
et al. Alpelisib
for PIK3CA-mutated, hormone receptor-positive
advanced breast cancer.
N Engl J Med. 2019;380(20):1929-1940.
3. André F,
Ciruelos E, Juric D, et al. Alpelisib plus
fulvestrant for PIK3CA-mutated, hormone
receptor-positive, human epidermal growth factor
receptor-1–negative advanced breast cancer:
final overall survival results from SOLAR-1.
Ann Oncol. 2020;32(2):208-217.
4. Data on file.
Novartis Pharmaceuticals Corp; 2021.
5. Data on file.
Novartis Pharmaceuticals Corp; 2018.
6. Ciruelos EM,
Rugo HS, Mayer IA, et al. Patient-reported outcomes
in patients with PIK3CA mutated hormone
receptor-positive, human epidermal growth factor
receptor 2-negative advanced breast cancer from
SOLAR-1.
J Clin Oncol. Published online March 29,
2021. doi:10.1200/JCO.20.01139.
This is an international site for PIQRAY®
(alpelisib) and is intended for healthcare professionals
outside the US.
